Teacher's Overview
Summary
In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen spontaneously, such as the splitting of water molecules to produce hydrogen and oxygen gases.
Objective
Students learn about using an outside energy source to drive a chemical reaction.
Safety
Be sure you and the students wear properly fitting goggles.
Materials
- 9-volt battery
- Two metal thumbtacks
- Water
- Epsom salt (MgSO4 • 7 H2O)
- Clean, empty, clear and colorless plastic water bottle with cap with the label removed
- Scissors
- Black permanent marker
- Beaker or plastic cup
- Paper towels or modeling clay
Time Required
Part of one class period, approximately 10–15 minutes.
Demo Tips
Epsom salt can be purchased locally in drugstores, where it is sold as a laxative and as a material to add to a warm water bath for treating aches and pains. Plastic containers other than a water bottle can be used, as long as the container has a flat bottom for easy connection to the battery contacts and the plastic is thin enough to easily push the thumbtacks through. For example, small plastic condiment cups could be used.
Integrating into the Curriculum
This demonstration could fit into a unit on chemical reactions or thermochemistry.
Teacher Procedure

- Remove the cap from a clean, empty, clear and colorless plastic water bottle. Turn the lid over so that the top of the lid touches the two contacts of a 9-volt battery. Center the lid over the two contacts. Using a black permanent marker, make two dots on the inside of the lid, one over the center of each contact.
- Place the lid on a hard surface with the top of the lid facing up. Push a metal thumbtack into the top of the lid directly over one of the dots. Push a second thumbtack into the lid directly over the second dot. The two thumbtacks should not touch.
- Using scissors, cut off the top half of the bottle, so that it looks like a funnel. Screw the lid back onto the bottle.
- Show students the top half of the bottle, particularly the two metal thumbtacks pushed through the lid.
- Fill the bottom half of the cut water bottle approximately half full of water. Add about a teaspoon of Epsom salt. Swirl to stir until most of the salt dissolves.
- Pour the Epsom salt solution into the top half of the bottle (hold so the lid faces down).
- Ask a volunteer student to observe the solution, particularly the two points of the metal thumbtacks. Is there any evidence of a reaction occurring?
- Place the two metal thumbtacks so that each thumbtack touches one of the contacts on the 9-volt battery. Ask the volunteer to observe the solution again. Is there any evidence of a reaction occurring?
- The battery can be placed into the bottom of a beaker or clear, colorless plastic cup, held upright with paper towels or modeling clay. The top of the bottle can then be rested on top of the battery with more stability if the instructor wishes to show it up close to the rest of the class.
- What evidence is there that a reaction occurs when the battery contacts touch the metal thumbtacks?
Bubbles of one or more gases are produced and rise from each of the thumbtacks.
- Is there any difference in the a mount of gas produced at each battery contact?
Yes, more bubbles are produced at one of the connections.
- Could the difference described in question 2 be due to a difference in the tacks? What could one do with the apparatus to potentially provide support for the idea that the battery contact is responsible.
One could rotate the battery contacts so that each contact is now touching the opposite thumbtack. The battery contact that produced more bubbles the first time should still produce more bubbles when touching either thumbtack.
- What is the purpose of the battery?
The battery drives a reaction that we did not observe happening spontaneously without this additional outside energy source.
- Why is Epsom salt added to the water?
Epsom salt is an electrolyte and serves to conduct electricity through the solution. Electricity from the battery is able to travel through the solution and between the two thumbtacks.
6. What is the balanced equation for the breaking apart of water molecules?
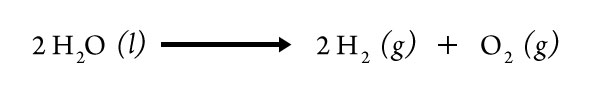
7. If the reaction we are observing is the breaking apart of water molecules, which battery contact might be producing which gas and why?
The reaction for the breaking apart of water molecules shows two gases being produced, H2 and O2. In the equation, twice as much hydrogen gas is produced than oxygen gas. The battery contact that produces more bubbles would be the one producing hydrogen gas.
8. What could be done to gather further evidence that there are two gases produced and that they are H2 and O2?
One could collect the gases produced and test them for known properties of H2 and O2 gases.
Separating the word “electrolysis” into its component parts summarizes its meaning—using electricity (electro-) to break apart (-lysis) something. In this demonstration, the electricity supplied by a 9-volt battery is used to break apart water molecules, overall producing hydrogen and oxygen gases.
The idea of including energy as a reactant or a product in a chemical equation can be used to illustrate the need for an additional outside energy force to drive a reaction that normally does
not happen spontaneously and to connect the demonstration to the concepts of exothermic and
endothermic reactions.
For example, Chemistry in the Community describes:
…if a particular chemical reaction is exothermic (releasing thermal energy), then the reverse reaction is endothermic (converting thermal into potential energy). For example, burning hydrogen gas—involving the formation of water—is exothermic. The energy released by formation of H–O bonds in water molecules is greater than that required to break bonds in H2 and O2 molecules:
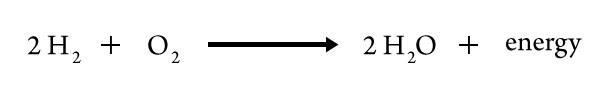
Therefore, the separation of water into its elements—the reverse reaction—must be endothermic, the quantity of energy equal to the quantity released when water is formed from gaseous H2 and O2.
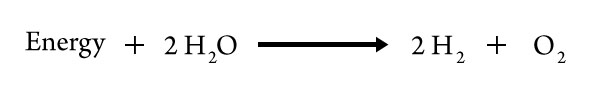
Challenge students to design a method for collecting the gases in the demonstration and to describe how they could be tested to determine if they are H2 and O2, and which is which. Glass tubes filled with the Epsom salt solution can be inverted over the two thumbtacks to collect the gases; it can take a substantial amount of time to collect an appreciable amount of gas.
Showing the reverse reaction, that of mixing hydrogen and oxygen gases and combusting them, could be used in connection with this electrolysis demonstration. This explosive, exothermic reaction should be used with appropriate safety precautions and equipment.
Another common setup used for showing water electrolysis uses two graphite pencils sharpened on both ends as electrodes.
Directions written for students to carry out this investigation are available for free in the American Chemical Society “Middle School Chemistry” curriculum.
