Teacher's Overview
Summary
In this investigation, students will explore basic thermodynamic concepts, including spontaneity, entropy, and enthalpy through a series of guided questions and procedures.
Objective
Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a deeper understanding of how ΔG = ΔHsys – T ΔSsys expresses the second law of thermodynamics by exploring energy transfer between system and surroundings as salts dissolve.
Safety
- Be sure you and the students wear properly fitting goggles.
- Ammonium chloride can be an irritant to body tissues. In the event of contact, wash affected areas with water.
- Ammonium nitrate is a strong oxidizer. May emit toxic vapors of NOx and NH3 when heated to decomposition. Can be an irritant to body tissues. In the event of contact, wash affected areas with water.
- Calcium chloride can be an irritant to body tissues. In the event of contact, wash affected areas with water.
- Acetone is flammable. Avoid flames or sparks. Irritating to body tissues. Avoid body tissue contact. Slightly toxic by ingestion. Skin contact causes dermatitis. Vapor may cause weakness, fatigue, nausea, and headache. Work in a well-ventilated area.
- Dispose of solutions according to local regulations.
Materials for Each Group
- 10 g ammonium chloride or ammonium nitrate
- 5 g calcium chloride
- Thermometer
- 100-mL graduated cylinder
- 3 150-mL beakers, stirring rod Optional Materials for the Post-Lab Demo
- Digital thermometer
- 10-mL graduated cylinder
- 10 mL acetone
Time Required
One class period, approximately 45–50 minutes.
Lab Tips
This lab is designed for students to work together, discussing and answering the questions posed while proceeding through the step-by-step treatment of the second law.
Pre-Lab Discussion
What does spontaneous mean? What kinds of processes in your experience happen spontaneously? Are there any differences between them?
Incorporating into the Curriculum
This investigation could be incorporated into a unit on chemical changes or thermodynamics.
Student Investigation
Thermodynamics is a way of describing energy transformations when a system changes from one state to another. The entire architecture of thermodynamics is built on carefully defined terms, many of which have an everyday meaning that is not exactly what chemists mean when they use the term. For example, one way that chemists state the second law of thermodynamics is that in any spontaneous change, the entropy of the universe increases. The underlined words have a very particular meaning that we need to know before we can understand the second law.
The second law of thermodynamics may be expressed in many ways, and it has been used by chemists to understand everything from the work of a steam engine to the direction of time. It grew in the nineteenth century out of observations made about big things like steam engines, and today it is often used to illuminate the conceptual, chemical world of tiny things like atoms, ions, and molecules. In this activity we will use careful observations of the process of dissolving salts in water to more deeply understand the second law.
What is a Spontaneous Change?
A spontaneous change is any change that happens freely in time. For example, you can drop a ball from above your head and it falls to the floor (spontaneous) but you need to provide energy to the ball to place it over your head again. Being able to predict what processes will be spontaneous is how we apply the second law.
Which of the following processes are spontaneous?
- Ice melts when dropped in a cup of warm water.
- Water evaporates when it is spilled on a hot surface.
- Water in a glass on your desk decomposes to hydrogen and oxygen.
- Iron rusts in air.
- The smell of perfume spray spreads throughout a room.
- Equal volumes of olive oil and vinegar dissolve together to make a salad dressing.
Good to know: spontaneous ≠ instantaneous! Even if a change is spontaneous, this doesn’t necessarily mean that it happens quickly. The second law tells us that all diamonds are spontaneously turning into coal, but this process is so slow we will never observe it taking place.
System, Surroundings, Universe
The system is the specific part of the universe we are considering, where a change is taking place. It can be any size—a test tube, a beaker, a human body, or an ocean. The surroundings are everything outside the system. The universe consists of the system and the surroundings together.
Fill in the blanks below. Label the regions with the terms system, surroundings, and universe.
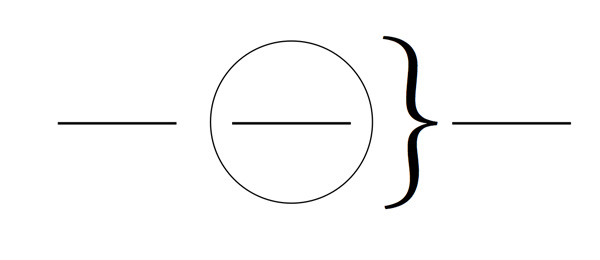
- Place 5.0 g of ammonium nitrate in a 150-mL beaker, then put the beaker on the portion of the diagram above that you have labeled as the system. Write the formula for ammonium nitrate and identify its solid type.
Formula:
Identify by underlining: Ionic Solid Covalent Solid Metallic Solid
- Record your observations of the macroscopic properties of the salt:
- Complete the sentence: Ammonium nitrate is an __________________ solid, and the individual particles in its lattice structure are ______________.
Defining Entropy and Looking at Entropy Changes in a System
Entropy is a mathematically defined property in thermodynamics. It can often help to understand it as a measure of the possible arrangements of the atoms, ions, or molecules in a substance. The symbol for entropy is S, and a change in entropy is shown as “delta” S or ΔS. If the entropy of a system increases, ΔS is positive. If the entropy of a system decreases, ΔS is negative.
- Pour 100 mL of water over the salt in the beaker and stir. Can you still see the ammonium nitrate?
- Make and record your observations:
- Using the terms cation, anion, solute, solvent, and solution, label the diagram below.
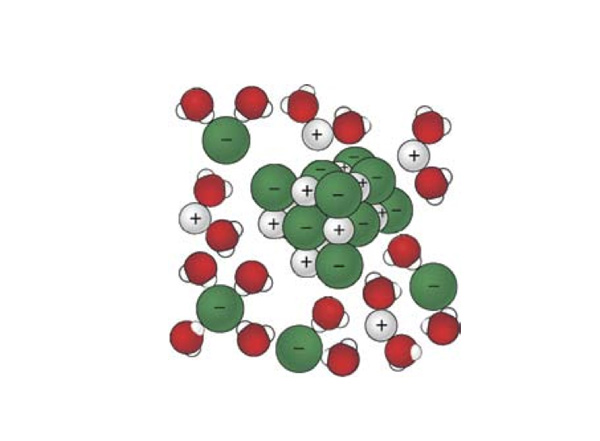
- Given the physical state of ammonium nitrate before it dissolves, how do the possible arrangements of the ions in the salt compare to their possible arrangements when free to move within the solution?
- Does your answer to the preceding item suggest that the entropy of the ammonium nitrate increased or decreased upon dissolving?
- Would ΔS for this change be positive or negative?
But more is going on than just ions leaving the solid and moving about more freely. Note in the figure above that the polar water molecules are attracted to and oriented around the dissolved ions. The ions are solvated. This orientation of a lot of the water molecules reduces their freedom to move about in the liquid, so the number of possible arrangements of the water molecules is reduced when the ions are present.
- Would ΔS for this change in the arrangement of water molecules be positive or negative?
The change in entropy for the reaction system ΔSsys has to include both the positive change for the ions and the negative change for the water molecules. Which one predominates? For most salts with single charges on their cations and anions, like NaCl, KBr, or LiNO3, the positive change in entropy for the ionic solid separating into its ions in solution will predominate.
- Did dissolution of ammonium nitrate happen spontaneously?
- If the overall ΔSsys for the dissolution of ammonium nitrate is positive, which ΔS in the system predominates: ΔS for the ions, or ΔS for the water molecules?
Looking at Entropy Changes in the Surroundings by Defining Enthalpy
We will now consider entropy changes in the surroundings by looking at another thermodynamic term, enthalpy. The enthalpy of a system has a definition in thermodynamics that relates to its internal energy, the pressure on the system, and the volume of the system. It is useful in understanding the second law, however, because at constant pressure and volume, a change in enthalpy is the same as the thermal energy transferred from the system to the surroundings, or from the surroundings to the system. The symbol for the enthalpy of a system is H, and a change in enthalpy is shown as “delta” H or ΔH. If thermal energy transfers from the system to the surroundings during a physical or chemical change, the ΔH is negative and the change is exothermic. If thermal energy transfers from the surroundings to the system during a change, the ΔH is positive and the change is endothermic.
Case one for enthalpy
- Measure out 100 mL of water in a clean 150-mL beaker. Once again, label the “system” and the “surroundings” in the diagram below.
- Place the beaker on your paper in the region labeled “system” below, then measure the temperature of its contents with a thermometer and record.
- Add 5.0 g of ammonium chloride or ammonium nitrate.
- While holding the beaker at its base, stir, and make and record your observations, including the final temperature of the mixture.
- Your hand at the base of the beaker can be considered part of the surroundings. Was thermal energy transferred to your hand from the beaker, or away from your hand to the beaker?
- Label the diagram below with an arrow, showing the direction of the transfer of thermal energy. Is thermal energy transferred from the surroundings to the system, or from the system to the surroundings?
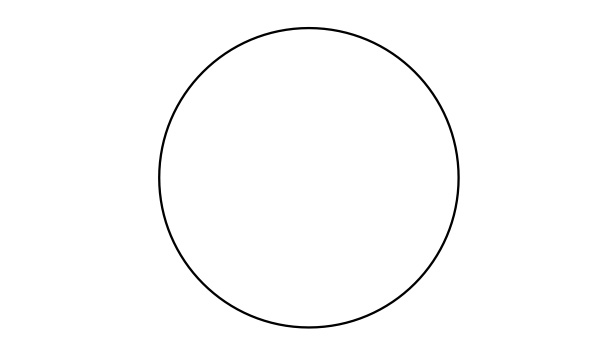
- Is the change endothermic or exothermic?
- Is ΔH of the system positive or negative?
Case two for enthalpy
- Measure 100 mL of water in a clean 150-mL beaker.
- Place the beaker in the “system” below, and measure and record the temperature of its contents. Add 5.0 g of calcium chloride.
- While holding the beaker at its base, stir, and make and record your observations, including the final temperature of the mixture.
- Was thermal energy transferred to your hand from the beaker, or away from your hand to the beaker?
- Label the diagram below with an arrow, showing the direction of the transfer of thermal energy. Is thermal energy transferred from the surroundings to the system, or from the system to the surroundings?
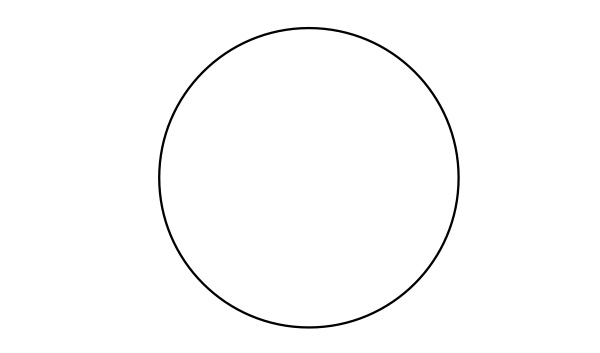
- Is the change endothermic or exothermic?
- Is ΔH of the system positive or negative?
Relating the change in enthalpy (ΔH of the system) to a change in entropy of the surroundings (ΔS of the surroundings)
We are going to look at how entropy changes in the surroundings, depending on the sign of ΔH of the system. Before we do, however, let’s review.
- When thermal energy is transferred from region A to region B, the molecules in region B, on average, ___________________ (speed up or slow down?).
- When the average speed of the molecules in a region is high, there are _____________ (more or fewer?) high-speed molecules. When the average speed of the molecules in a region is low, there are ________________ (more or fewer?) high-speed molecules.
- When the average speed of the molecules in a region is high, there are _____________ (more or fewer?) possible arrangements for the molecules among the molecular speeds. When the average speed of the molecules in a region is low, there are ______________ (more or fewer?) possible arrangements for molecules among the molecular speeds.
Now consider what happens in the surroundings during the process of thermal energy transfer.
- When thermal energy is transferred in an endothermic change, will the molecules of the surroundings speed up or slow down?
- How will this affect the entropy of the surroundings, will it increase or decrease?
- In this case, is ΔS of the surroundings positive or negative?
- When thermal energy is transferred in an exothermic change, will the molecules of the surroundings speed up or slow down?
- How will this affect the entropy of the surroundings, will it increase or decrease?
- In this case, is ΔS of the surroundings positive or negative?
Looking at entropy changes in the universe and the second law
In any spontaneous change, the entropy of the universe increases, or ΔS universe > 0. We have seen that we can consider the entropy of the system and the entropy of the surroundings separately. Since the universe is composed of the system and the surroundings, then we will consider both when determining the change in entropy of the universe:
ΔS universe = ΔS surroundings + ΔS system
- When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe must be positive, so the reaction will always be spontaneous, under the given conditions.
- If both terms are negative, the reaction is never spontaneous under the given conditions.
- If one term is positive and the other is negative, then whichever has the largest absolute value determines whether ΔS universe is positive (spontaneous reaction) or negative (reaction is not spontaneous) under the given conditions.
Complete the following:
- In an exothermic reaction, ΔS surroundings is _____________ (positive or negative?).
- In an endothermic reaction, ΔS surroundings is ____________ (positive or negative?).
- In a spontaneous, endothermic reaction, thermal energy is spontaneously __________________(absorbed or released?) by the system from the surroundings.
- In a spontaneous, exothermic reaction, thermal energy is spontaneously __________________ (absorbed or released?) to the surroundings from the system.
Application of Free Energy
Thermodynamics also defines a term known as free energy, G; the change, ΔG, when a system undergoes a change, is often considered the energy of the system available to do work. Free energy also relates to the second law, since in any spontaneous process the change in free energy (ΔG) for the system is negative. The definition for the change in free energy summarizes our discussion of entropy changes in the system and in the surroundings:
ΔG = ΔHsys – T ΔSsys
We have seen that whenever ΔHsys is negative (exothermic) the ΔSsur increases, or is positive.
- So if ΔHsys is negative and ΔSsys is positive, what will the sign of ΔS universe always be?
- Under these conditions, is the reaction spontaneous?
- And under these conditions, what will the sign of ΔG always be?
This famous relationship of free energy change to changes in enthalpy and entropy shows us the balance between entropy changes in the system and the surroundings, and how that balance depends on temperature. Note that T stands for the absolute temperature in Kelvin, so its value is always positive.
Use the relationship ΔG = ΔHsys – T ΔSsys to complete the table below:
| If ΔHsystem is | Then ΔSsurroundings will be (+ or −) | And if ΔSsystem is | And ΔSuniverse will be (+ or −) | The ΔG will be (+ or −) | And the reaction is (spontaneous or not spontaneous) |
|---|---|---|---|---|---|
| negative or exothermic | positive (any temperature conditions) | ||||
| negative or exothermic | negative (any temperature conditions) | ||||
| negative or exothermic | negative (high temperature conditions) | ||||
| negative or exothermic | negative (low temperature conditions) | ||||
| negative or exothermic | positive (high temperature conditions) | ||||
| negative or exothermic | positive (low temperature conditions) |
Decomposition of Baking Soda
The mysteries of bread making began to be simplified in the 1800s with the use of baking soda. Although it was also combined with sour milk to lighten the texture of heavy bread, it could produce a lightening effect in bread dough on its own when it decomposed:
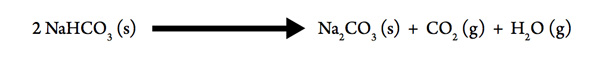
- Gases are produced in the decomposition, therefore the sign of ΔSsys for the process is likely to be ___________.
- Heat is absorbed in the process so the sign of ΔHsys is ___________.
- Apply this information to ΔG = ΔHsys – T ΔSsys . When is it likely that ΔG will be negative, in other words, when will the process be spontaneous, at high T or at low T? _______________
- If the values of ΔHsys and ΔSsys are found, we can predict the temperature needed for the baking soda to decompose, and adjust our baking temperature accordingly!
Melting Point of Water
Consider the process:
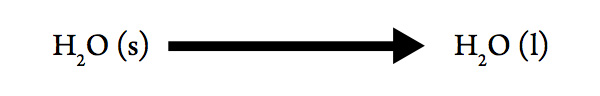
- Determine the sign for ΔHsys. Explain your choice.
- Determine the sign for ΔSsys. Explain your choice.
- Will ΔG be negative at high or at low temperatures?
- We can use ΔG = ΔHsys – T ΔSsys to predict the temperature at which a substance will melt, if we have values for ΔHsys and ΔSsys. At some temperature (T), ΔG will change from positive to negative. What will be the value of ΔG at the temperature of this change?
- Substitute this value for ΔG and rearrange ΔG = ΔHsys – T ΔSsys to solve for T. This value of T will be what?
Production of Ozone
Ozone (O3) is an unstable form of oxygen that is formed in the stratosphere. The ozone layer in the upper atmosphere protects life on the earth’s surface from high-energy ultraviolet light from the sun. Ozone is produced from oxygen gas:

- In this process, _______________ moles of oxygen gas produce ______________ moles of gaseous ozone. Are there more possible arrangements (higher entropy) for the reactants or the products? _______________Therefore, is ΔSsys positive or negative?
- The process is endothermic, so ΔHsys is _____________________.
- Use ΔG = ΔHsys – T ΔSsys to consider: At what T will the reaction be spontaneous?
- If a system will never spontaneously absorb thermal energy from the surroundings to undergo a change, does that mean the change is impossible? Could energy somehow be forced into the system, for example, by doing “work” on the system?
- In the case of the production of ozone in stratosphere, which goes on every day, what kind of energy could be “working” on the oxygen gas?
Decomposition of Hydrogen Peroxide
If you have ever used hydrogen peroxide to disinfect an open cut, you may have seen bubbles form when the hydrogen peroxide decomposes:
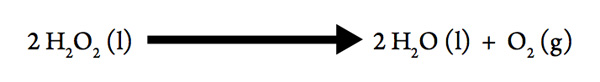
- What must be forming in the bubbles?
- When a gas is formed from a liquid, is the sign of ΔSsys positive or negative?
- This decomposition is exothermic, so what is the sign of ΔH?
- So the decomposition of hydrogen peroxide is spontaneous at _________temperatures.
- Consider that the bottle you bought from the store has been sitting on the shelf for some time, and still contains H2O2. Does the conclusion that a reaction is spontaneous at all temperatures mean that the reaction happens quickly? What could have caused the reaction to speed up when the hydrogen peroxide was applied to your open cut?
Demo
- Place the measuring end of a digital thermometer into a 10-mL graduated cylinder full of acetone.
- Have students note the temperature, then remove the thermometer, waving it a bit in the air to rapidly evaporate the acetone. The temperature quickly falls.
- Have students analyze the process from the point of view of thermodynamic properties (the endothermic ΔHvap, the positive ΔSsystem, and the spontaneity of the process).
Related Content
You can explore the basic mechanics of evaporative cooling in the investigation The Energy of Evaporation.
Practice Problem
Now that we’ve observed the qualitative relationships between ΔH, ΔS, and ΔG, let’s calculate the quantitative value of ΔG°rxn for the dissolution of solid ammonium nitrate in water at 25 °C:
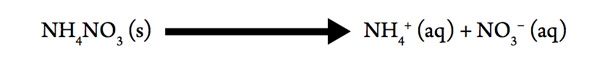
| ΔH (kJ/mol) | So (J/mol K) | |
|---|---|---|
| NH4NO3 (s) | −365.6 | 151 |
| NH4+ (aq) | −132.80 | 112.8 |
| NO3− (aq) | −206.57 | 146.4 |
Remember that ΔH°rxn = Σ n ΔH°f (products) − Σ n ΔH°f (reactants) and ΔS°sys = Σ n So (products) − Σ n So (reactants). Then use ΔG = ΔHrxn – T ΔSsys to find the change in free energy. Pay attention to units as ΔH°rxn will be calculated in kJ and ΔS°sys will be calculated in J. Be sure to reconcile units before finding ΔG.
- ΔH°rxn =
- ΔS°sys =
- ΔG°rxn =
Do your lab observations support your results? Explain.
