Teacher's Overview
Summary
Students use a Bunsen burner, microwave oven, and hot plate to determine which instrument heats water most efficiently. Students perform detailed calculations to support their conclusions.
Objective
Students will use observations to calculate the energy efficiency of various heating methods.
Safety
- Be sure you and the students wear properly fitting goggles.
- Review safe use of Bunsen burners or hot plates. Caution students about handling hot
water to avoid scalds or burns. - Methane (natural gas) is flammable. It can be explosive if mixed with air in certain proportions. Avoid any sparks or flames when collecting the gas. Methane is toxic by inhalation. Work in a well-ventilated area.
Materials for Each Group
- 1 Bunsen burner
- 1 hot plate
- 1 one-meter hose sections of tubing
- 1 tub or bucket for holding water
- 1 stopwatch or clock with a second hand
- 1 2-L soda bottle (clear with label removed)
- 1 400-mL beaker
- 1 alcohol thermometer
- 1 ring stand support with ring
- 1 pair of beaker tongs
Materials for the Whole Class
- 1 microwave oven (shared by all groups)
- A variety of volumetric containers, such as large graduated cylinders for each group
Time Required
Two to three class periods, approximately 45–50 minutes each.
Lab Tips
It may be difficult for some students to come up with a satisfactory scheme for collecting and measuring the amount of methane collected from the gas outlet. Lend appropriate hints and tips as required. If your lab doesn’t have a typical standard gas system piped in permanently, consider using portable gas burners, alcohol lamps, or whatever other alternative system you generally use. Also, consider using portable electric immersion heaters in the place of hot plates. They are much more efficient and at $10 per heater, much less expensive.
If presented as a “lab challenge” this basic investigation could be adapted as a lab practical exam to test how students are able to apply what they have learned. You could vary the complexity of the task by adjusting the amount of information you provide (such as the enthalpy of combustion for methane). This investigation also provides an opportunity for considering the total environmental cost involved in a simple lab procedure.
Pre-Lab Discussion
Make sure students are familiar with the proper operation of Bunsen burners, hot plates, and microwaves. You may direct students to read the procedure and make up appropriate data tables before they go to the lab. Their data tables can be their “passport” to begin the investigation.
Incorporating into the Curriculum
This investigation could be incorporated into a unit on stoichometry, chemical changes, or thermochemistry.
Student Investigation
Taking into account the energy requirements for a process and finding ways to minimize the energy required are important ways to make the process as environmentally friendly or “green” as possible.
One way to do this is to make the process as efficient as possible. But what do we mean when we say efficient? Usually it means the fraction or percent of all the energy that goes into a process that actually is used for the desired effect; the rest is “lost.” Although we say “lost,” we know that energy is never lost, it just moves out of the system we are interested in or gets converted into a less useful form.
Consider a simple example that does involve energy. Suppose our process is to catch the water leaking from a pipe in our house. If 1000 mL of water leaks and our container holds only 650 mL, we would say our catchment process is 65% efficient. That is, because

was caught in the container. The other 35% was “lost.” Of course, this 35% wasn’t really lost either, it is on the floor under the leak, but it was “lost” to our system (the container). In this lab you will consider the energy efficiency of heating water in a typical high school laboratory setting. You will consider three ways this is typically done and calculate the efficiency of each method.
Part A—Heating with a Bunsen Burner
One common way to heat water in the laboratory is to use a Bunsen burner. The typical setup is to use natural gas that is piped into the classroom from a commercial supplier and mixed with air in the burner to produce a flame. Mixing with air ensures a more complete combustion. Natural gas is composed primarily of methane (CH4). The complete combustion of methane is represented in the following equation:
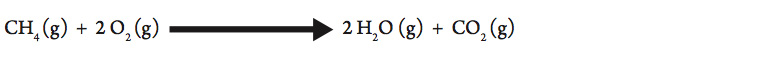
This reaction is very exothermic (gives off a lot of thermal energy).
Of course, not all of the energy released by this reaction is actually absorbed by the material being heated. Some of the energy goes into warming the container or the immediate surroundings. The efficiency of the process can be calculated as follows:
How efficient is it to heat a sample of water using a Bunsen burner?
- Using the balanced equation above, calculate the amount of energy (ΔH) for the combustion of methane. (Assume we are at STP.)
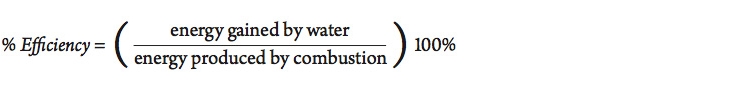
| Methane (CH4) | −74.85 | Water (H2O) | −241.8 |
|---|---|---|---|
| Oxygen (O2) | 0 | Carbon Dioxide (CO2) | −393.5 |
The equation for finding the standard enthalpy change of a chemical reaction is:
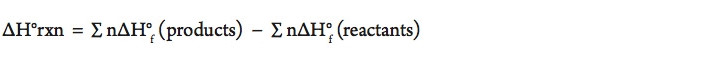
- Devise a method to determine the rate (L/s) natural gas is delivered from a fully opened gas valve connection on your desktop, through the Bunsen burner. You will have access to the following equipment: 1-meter hose, a tub or bucket for holding water, a stopwatch or clock with a second hand, a 2-L soda bottle, and a variety of volumetric containers, such as large graduated cylinders.
- Write a plan, paying particular attention to safety, including how you will safely dispose of the natural gas you are measuring. When you have documented the plan, show it to your teacher and get approval before proceeding. Be sure to record your results during the procedure for use in completing this activity.
- Fill a beaker with a carefully measured (+ or − 1 mL) amount of tap water (somewhere between 175 and 225 mL). Set the beaker on a ring stand or support suitable for heating it with a Bunsen burner. Measure the initial temperature of the water with a thermometer to the nearest 0.1 °C, and begin to heat it with the Bunsen burner. Be sure the stopcock of the gas fitting is wide open, as before. Start tracking the time.
- Heat the water until the temperature rises by 30–50 °C. Measure the final temperature to the nearest 0.1 °C. Note the time elapsed during the heating.
Part B—Heating with an Electric Hot Plate
Another typical means of heating in the laboratory is to use an electric hot plate. In this part you will do an experiment similar to the one described in Part A, but this time you will determine the efficiency of using an electric hot plate to heat water.
- In this case, the energy released b 1. y the hot plate depends on its energy rating. Look at the bottom or sides of the hot plate for its power rating, measured in watts.
- Since a watt = 1 J/s , we can calculate the total amount of energy released from the hot plate using the following equation:
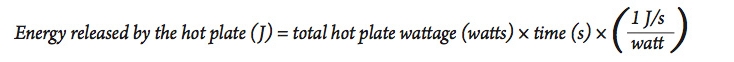
- Using a procedure similar to the activity above, heat a sample of water similar in size to the amount used in part A, and use the results to calculate the efficiency of the electric hot plate for heating water. Be sure to use the hot plate on the highest setting to be sure it is operating at its highest power output.
Part C—Heating with a Microwave Oven
Microwave ovens have long been used in homes, but now they are being used more widely in scientific laboratories. Use the experience and information in the previous two investigations to determine the efficiency of heating a sample of water with a microwave oven.
- Look at the bottom, sides, or elsewhere on the microwave oven for its power rating, measured in watts. This information is usually on a small sticker or plate.
- Since a watt = 1 J/s , we can calculate the total amount of energy released from the microwave using the following equation:

- Using a procedure similar to the activity above, heat a sample of water and use the results to calculate the efficiency of the microwave oven for heating water. Be sure to use the microwave oven on the highest setting to be sure it is operating at its highest power output. It is best to not leave the thermometer in the microwave while it is operating. Stop the microwave and take the temperature at intervals until the desired temperature change is achieved.
Analyzing Part A
Calculate the amount of heat absorbed by the water, the amount of heat released by the burning natural gas, and the percent efficiency of the heating process. Use the following equations in your calculations (assume STP).
The equation for determining the amount of thermal energy absorbed by a substance (where a change in state is not involved) is given by the equation:

In our investigation, the energy absorbed by the water is given by the equation:
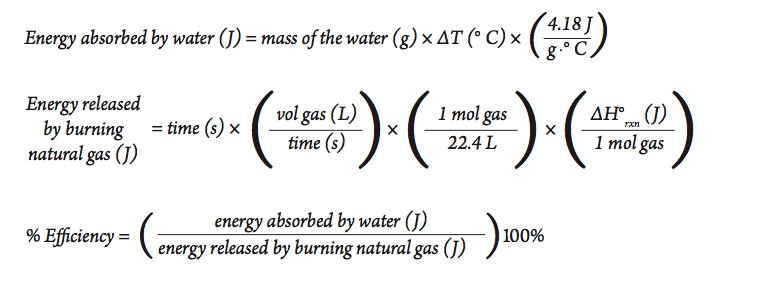
Analyzing Part B
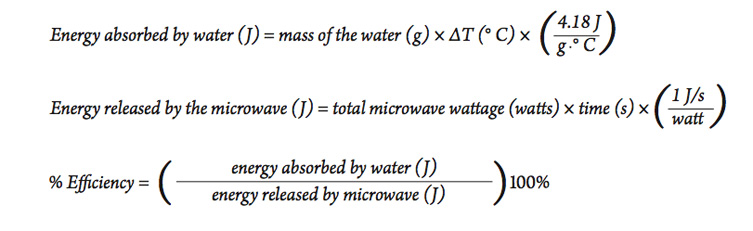
Analyzing Part C
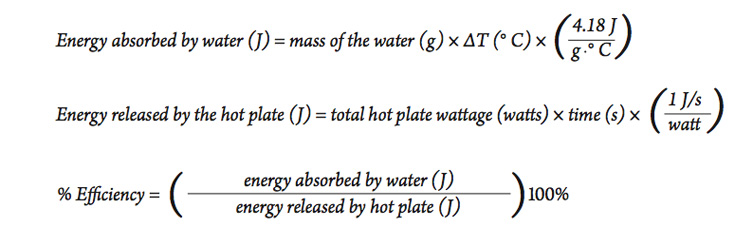
- When you have calculated the efficiency for heating by each method, record your results, along with the rest of the class, on the board in the front of the classroom, or as your teacher directs. Calculate the average efficiency for each of the methods and discuss the precision in the range of results of the data from the various lab groups. Discuss any result that differs significantly from the class average, and if time allows, repeat the experiment to improve the precision.
- Compare the results of the three methods of heating. Which was the most efficient, and which was the least efficient?
Practice Calculations
You can get more practice with calculations like these in Preparation & Combustion of Biodiesel.
The data you gathered helps determine the efficiency of the various types of heating, but does not reveal the total cost of heating. Depending on how it is generated, the power plants that produce electricity may contribute significantly more (or less) pollution in the process than does the production of natural gas.
The total cost of energy should reflect not only the cost of production, but also the indirect costs associated with damage to the environment, regulations, and other factors.
- Use your household electricity and natural gas bills to calculate the cost in dollars for each part of this investigation. Calculate the cost for heating a 200-g sample of water by 10 °C for each type of heating. If natural gas is not available in your community, use the national average price or substitute the cost of propane gas (an alternative fuel to natural gas).
This cost in dollars is rarely a reflection of the total cost to the environment, since it only reflects the net cost (after government subsidies) to consumers for the energy. Which energy source was the most expensive? Which was the least expensive?
- There are many ways to generate electricity and produce natural gas. Some require lessenergy to produce, and some give off less pollution. Using the Internet and other resources, investigate which power source tends to require less energy to produce and contributes less total pollution to the environment.
- Using the information you collected regarding efficiency, dollar costs, and environmental costs, make a recommendation for how best to minimize the energy used to heat substances in your school laboratory. Explain the reasoning behind your recommendation.
