This student reading is courtesy of ChemMatters, the ACS quarterly magazine division that explores chemistry behind everyday life. Learn more about ChemMatters, view free online content, or subscribe at acs.org/chemmatters.
Pop Quiz!
Question: What happens when an ice cube is added to warm soda?
- Heat is transferred from the soda to the ice.
- The ice cube’s “cold content” is transferred to the soda.
- Both heat and cold are transferred between the ice and soda.
In our daily lives, we see many examples of warm objects that become colder, but it may not be obvious how it happens. Let’s see how things become cold (and get the answer to our pop quiz).
Collisions in your soft drink
In the case of the ice cubes in the soft drink, the soft drink becomes colder and the ice cubes warmer because energy is transferred from the soft drink to the ice, not the other way around, so the best answer is “a”. The reason is that energy is always transferred from a body at higher temperature to a body at lower temperature and, as a result, there is no such thing as “cold” flowing from the ice to the drink.
To understand how this works, let’s consider the definitions of “temperature” and “kinetic energy.” In any sample of matter, particles—atoms, ions, or molecules—can move in three different ways: They can vibrate (wiggle about a fixed position), translate (move from one location to another), or rotate (spin around).
At any time, these particles are constantly moving—slowly in solids, and more noticeably in liquids and gases, and as they do, they collide with one another. Each time these collisions occur, the speeds and directions of the colliding particles change. So the particles have a wide range of speeds and, similarly, a wide range of kinetic energies (because kinetic energy is proportional to the square of the speed). The values of the kinetic energies of all the particles follow a distribution that looks like the one shown below.
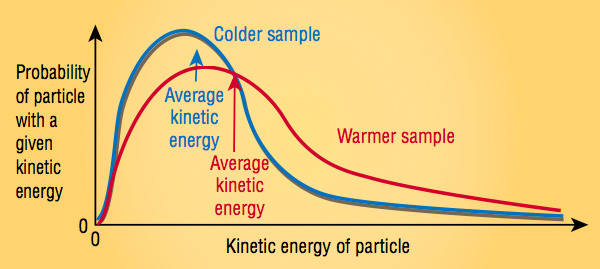
So a common way to discuss the kinetic energy of all the particles in a sample is to use their average kinetic energy, which lies toward the middle of the distribution. This average kinetic energy is proportional to temperature, so temperature is a measure of this average kinetic energy. The more the particles vibrate, translate, and/or rotate, the greater the temperature of the sample.
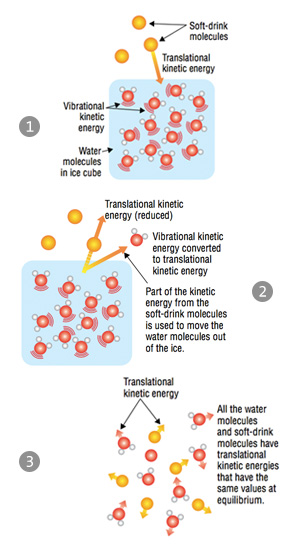
Let’s return to the example of ice in a warm soft drink. The particles in the soft drink move throughout the space of the container, colliding with each other and with the walls of the container. This is known as translational kinetic energy, and it is the main form of kinetic energy for gases and liquids. The water molecules in the ice vibrate about a fixed position and, as a result, they have a “vibrational” kinetic energy. The molecules in the soft drink move faster than the water molecules in the ice cubes because they are at a higher temperature and, therefore, have a higher average kinetic energy than the water molecules.
So what does the energy transfer look like? When the faster-moving soft-drink molecules collide with the slower-moving water molecules, part of the kinetic energy of the soft-drink molecules is used to move the water molecules out of their crystal lattice. The net effect is that the kinetic energy of the soft-drink molecules is reduced and the water molecules have the same kinetic energy that they initially had in the ice crystal, except that this kinetic energy is translational rather than vibrational. The total amount of energy transferred from the soft-drink molecules to the water molecules in the ice cubes is called heat.
The collisions will continue to transfer energy until the temperatures of the ice molecules and soft-drink molecules are identical. This is known as thermal equilibrium. When thermal equilibrium has been reached, the average kinetic energy of both types of particles is equal, and the final temperature—assuming there is still ice left—will be about 0 ºC. At thermal equilibrium there is no change in the average kinetic energy of either sample; collisions still occur, but there are an equal number of collisions resulting in an energy gain as there are collisions resulting in an energy loss.
Cold finger
Thinking about heat and cold in this way can be useful in explaining how heat transfer works in everyday life. It can help us explain common occurrences such as evaporation.

If you put your finger in water at the same temperature as your finger and then wave it in the air, it feels cooler. Why? Some of the water molecules with higher energy have enough energy to escape as water vapor, leaving behind water molecules with a slightly lower average energy, or a little lower temperature. Molecules in your finger collide with the molecules of water and transfer some of their kinetic energy to the water, thus slightly lowering the finger temperature. This is heat transfer due to the evaporation of water molecules on the surface of your skin.
This happens over and over again, as higher energy water molecules vaporize, leaving water molecules with lower average kinetic energy, so heat transfer continues from your finger to the cooler water molecules. This net transfer of energy goes from your finger to the water, and your finger feels cooler.
So, does cold exist? Can we say that it is really cold outside? Yes, we can. Cold is a perfectly fine adjective to describe when something is not hot, or when its temperature is low. We sometimes use words to describe conditions that reflect the absence of something rather than the presence of something else. For example, there is no such thing as darkness. There is only light or lack of light. But that’s a story for another time!
Download Article

