This student reading is courtesy of ChemMatters, the ACS quarterly magazine division that explores chemistry behind everyday life. Learn more about ChemMatters, view free online content, or subscribe at acs.org/chemmatters.
April 16, 1947
It started with a small fire on the S.S. Grand Camp, a French cargo ship anchored off Texas City, TX. The ship had recently taken on 2380 tons of ammonium nitrate (NH4NO3) fertilizer.

Early efforts by the crew to extinguish the fire were unsuccessful. The fire soon raged out of control. At 9:12 a.m., the ammonium nitrate exploded, sending the 7200-ton ship 20 feet in the air—the first of a series of catastrophic events. Burning debris reached surrounding oil refineries and chemical plants. A 15-foot tidal wave caused two other ships anchored in the harbor to collide. Both were soon ablaze. One of impacted vessels also contained ammonium nitrate. By the time the last flame had been extinguished, 576 people were dead and Texas City was in ruins.
Early efforts by the crew to extinguish the fire were unsuccessful. The fire soon raged out of control. At 9:12 a.m., the ammonium nitrate exploded, sending the 7200-ton ship 20 feet in the air—the first of a series of catastrophic events. Burning debris reached surrounding oil refineries and chemical plants. A 15-foot tidal wave caused two other ships anchored in the harbor to collide. Both were soon ablaze. One of impacted vessels also contained ammonium nitrate. By the time the last flame had been extinguished, 576 people were dead and Texas City was in ruins.
April 19, 1995
It was one of the most devastating acts of domestic terrorism ever to hit our nation. A truck loaded with roughly two tons of a mixture of ammonium nitrate fertilizer and fuel oil was detonated with a blasting cap on a street just outside the Alfred P. Murrah Federal Building in Oklahoma City, OK. A total of 168 people, many of them children, lost their lives. In 1997, two U.S. citizens, Timothy McVeigh and Terry Nichols, were convicted. McVeigh was sentenced to death and executed on June 11, 2001. Nichols is serving a life sentence.

Ever since Alfred Nobel, the founder of the Nobel Peace Prize, developed a process to make dynamite in 1867, explosives have played a key role in both peace and war.
Today, when we think of explosives, substances like TNT (trinitrotoluene) and nitroglycerin come to mind. But ammonium nitrate? That’s just a simple fertilizer! What is it about this simple inorganic compound that can cause it to react so violently? As you probably guessed, the answer is in the chemistry.
All explosions share some features. They all involve the rapid and violent release of large amounts of energy from a confined region of space. Particularly true for chemical explosions, they often involve the rapid expansion of gases generated during the explosion itself. Chemical explosions like those in Texas City and Oklahoma City are accompanied by a loud sharp report, flying debris, heat, light, and fire.
An explosive is a chemical compound or mixture that does the job. The explosive decomposition of nitroglycerin illustrates several features common to explosions:
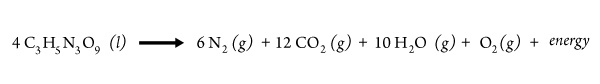
First, the reaction is exothermic, meaning that it releases energy. Second, it produces several gaseous products, all of which expand as the released energy raises the temperature. Third, even though the equation doesn’t show it, the reaction is very rapid—once underway, all the energy is released in a very short time. Finally, the reactants include the element nitrogen.
Why do so many explosives contain the element nitrogen? Look at the products of explosive reactions and you’ll find the same gasshowing up over and over—ordinary nitrogen gas, N2. The irony is that nitrogen gas is a very stable compound at a very low energy state. But when it is formed from reactants that start out in a very high energy state, a very large amount of energy is released in the process. Kaboom!
Why do explosive compounds react so rapidly? One way to speed up a reaction is to thoroughly mix
the reactants. Mixing allows for immediate contact to occur. You may have read about explosions in flour mills and grain elevators. Even otherwise harmless substances like flour can explode violently if thoroughly mixed with air and ignited by a spark.
Molecules of explosive compounds like nitroglycerin or trinitrotoluene take the mixing step one step further. For these compounds all of the reactants are on board the same molecule. Immediate contact is assured.
Let’s go back to the Texas City tragedy. What caused the ammonium nitrate in the holds of the ship to explode without the use of some other explosive? Chemists found that the answer was in the bag. The ammonium nitrate fertilizer was packaged in plain paper. The cellulose used to make paper contains a large amount of the element carbon. It was the carbon and ammonium nitrate mixture that reacted to unleash
the tragic explosion.
By analyzing the circumstances surrounding the Texas tragedy, chemists began to appreciate the power and potential of ammonium nitrate-based explosives. An effective, relatively safe, and inexpensive explosive called ANFO (ammonium nitrate fuel oil) was developed. And there were no risky transport problems to be solved. To make ANFO, ammonium nitrate and fuel oil were mixed at the blast site.

But ANFO was limited as a commercial explosive. Ammonium nitrate is water-soluble. As it gains water, the energy necessary to initiate its reaction with fuel oil increases to levels making it useless as an explosive. Dupont chemists went to work to produce a form of ammonium nitrate that would detonate even in a wet environment. By adding sensitizers, they were able to detonate the mixture with less-energetic shock waves. Then by adding thickening agents, they produced a syruplike mixture called TOVEX—easy to pour into drill holes at the blasting site. It’s interesting that dynamite’s inventor Alfred Nobel became a pacifist later in life. The man whose name will forever be known as the father of modern explosives dedicated much of his influence and fortune to opposing their use as weapons of destruction.
Download Article
A Nitrogenous Timeline
1867. Dynamite is invented.
Dynamite is invented. Nitroglycerin is a highly unstable liquid likely to explode with the slightest shock. To reduce its obvious hazards, Nobel uses a finely powdered silicon-based absorbent called Kieselguhr to soak up the liquid nitroglycerin—thus, stabilizing the explosive without sacrificing its strength. Later, he replaces Kieselguhr with sawdust and sodium nitrate. He substitutes ammonium nitrate for some of the nitroglycerin to make a new, lowcost explosive, dynamite.
1900. TNT production costs drop.
TNT is appreciated as a very stable solid that can be poured and even melted with relative safety.
1914. TNT is used as a weapon in World War I.
TNT’s big advantage over dynamite is its capacity for producing shock waves that can rupture the steel on armor-plated vehicles.
1940. World War II
weaponry introduces two new explosives, RDX (hexahydro-1,3,5-trinitro 1,3,5-triazine) and PETN (pentaerythritoltetranitrate). With additions of wax, motor oil, and other stabilizing fillers, RDX is renamed Composition Four, or C-4 explosive. Stable within a large temperature range (–70 ° to 170°F), safe to handle, and easy to mold due to it’s plasticlike properties, C-4 is attached to bridge supports, armored vehicles, or the hulls of ships. It is detonated with blasting caps.
1945. Ammonium nitrate
is manufactured, stockpiled, and shipped to war-torn Europe as an inexpensive fertilizer for enriching depleted farm soil. It’s potential explosive power is known, but its stability relative to other explosives seems adequate for production and storage.
1947. S.S. Grand Camp explodes in Texas City Harbor.
Chemists reconsider the stability of ammonium nitrate.
1957. ANFO
(Ammonium Nitrate Fuel Oil) explosive is developed by taking ammonium nitrate “prills” and mixing them with liquid fuel oil to make a “slush”. Mixed at the blast site, ANFO is relatively safe to handle.
1988. Pan AM Flight 103
wreckage is found to contain RDX residue. Presumably, as a result of terrorism, the plane crashed in Lockerbie, Scotland, killing all 270 people on board.

