This student reading is courtesy of ChemMatters, the ACS quarterly magazine division that explores chemistry behind everyday life. Learn more about ChemMatters, view free online content or subscribe at acs.org/chemmatters.
We have all used a thermometer—to check for a fever, record data during a chemistry lab, or to help us decide how to dress before leaving for school in the morning. But have you ever thought about how a thermometer works? And when you measure temperature, just what exactly are you measuring?
The prefix thermo- refers to heat. Thermodynamics is the study of heat. A thermos either keeps heat in or out. You wear thermal underwear to prevent body heat from escaping. Despite its name, however, a thermometer does not actually record heat, but rather temperature. Temperature and heat are two radically different concepts.

Temperature is a measure of the average kinetic energy of the molecules within a substance. When you record the temperature of something, you are making a statement about how fast the molecules are moving. When you are waiting for a bus in the morning in the middle of January, instead of saying, “Boy, its cold out here this morning,” it would be more accurate to say, “Boy, the molecules in the air are moving quite slow this morning!”
Heat vs. Temperature
Heat is a little trickier to define. Heat refers to the movement of energy from a substance of high temperature to one of low temperature. Heat always refers to energy in transit. A substance can have a high temperature, but little heat available to transfer. A drop of boiling water contains less actual heat than a bathtub full of water at a lower
temperature. Temperature is a measure of only the average kinetic energy of molecules, but because heat depends on the total energy, there is not a simple, universal relation between the two.
Here’s an everyday example that helps to illustrate the difference between heat and temperature. Consider ice: when you cool a drink using ice, a lot of heat flows from the drink into the ice (so the drink’s temperature falls). But the temperature of the ice does not rise, it stays at 0 °C—the heat goes into breaking the interactions between water molecules to melt the ice (at 0°) to form water (still at 0°). Ice and water at 0° have the same temperature but very different amounts of heat.
Temperature Scales
In the United States, most thermometers for everyday use are calibrated in degrees Fahrenheit. Most of the rest of the world measures temperature in degrees Celsius. At one point during the 18th century, there were nearly 35 different temperature scales in use! Many scientists felt the need to devise a uniform temperature scale that would meet widespread acceptance.
One temperature scale that met with some success was the Romer scale, which was first used in 1701. This temperature scale was invented by Ole Christensen Romer, aDanish astronomer whose biggest claim to fame was measuring the speed of light in 1676. His temperature scale set the boiling point of water at 60° and the freezing point at 7.5°. The lowest temperature you could achieve with a mixture of salt and ice was 0°. Because most people from that time period were not too concerned about the temperature of ice and salt, this scale was destined for the dustbin of history.
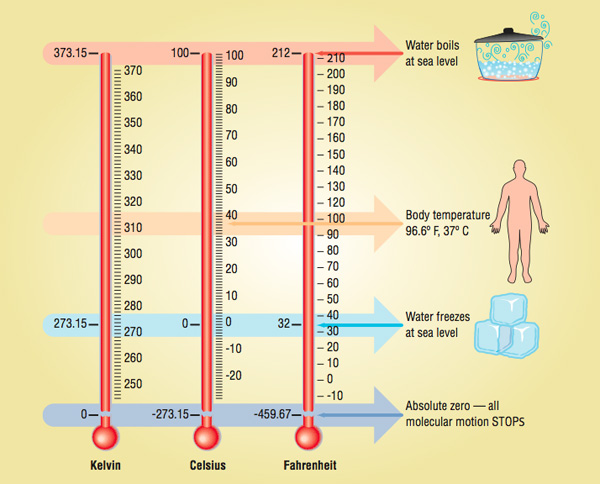
Daniel Gabriel Fahrenheit, a German physicist, published an alternate scale in 1724. Borrowing from the work of Romer, he set 0 °F as the lowest temperature that could be achieved with a mixture of salt, ice, and ammonium chloride. (It is unclear whether Romer also used ammonium chloride in his experiments, as many of his records were destroyed in a fire.) Fahrenheit set the freezing point of water at 32° and the body temperature of a person at 96°, which he determined by measuring the temperature under his wife’s armpit. Each degree of his scale corresponded to one ten-thousandth the initial volume of mercury used in his thermometer.To this day, there is considerable controversy as to how Fahrenheit actually arrived at his temperature scale. He never did reveal exactly how he arrived at the reference points for his thermometer, as he did not want others to construct and sell the thermometers he had spent much of his life perfecting.
His scale met widespread acceptance because everyone could relate to it, since 0 °F and 100 °F were the lowest and highest temperatures typically experienced on any type of regular basis in Western Europe. If the temperature rose above 100°, you knew it was really hot. If the temperature dipped below 0°, you knew it was quite cold. Whether these points were intentionally chosen to represent these extremes or just happened to work out this way is still being debated today. The biggest problem with this scale was the freezing and boiling points of water were set at 32º and 212°, not exactly round numbers. This was an issue not so much with the general public, but rather with scientists, who tend to obsess over such things. However, others have postulated that placing 180 degrees between the freezing and boiling points of water was not arbitrary but quite rational, as this number represents the number of degrees in half a circle.

To counter this problem, Swedish astronomer Anders Celsius came up with another scale in 1742, setting the freezing and boiling points of water at 0° and 100°, with 100 divisions in between. Hence, it was termed the Centigrade scale, since the prefix centi- represents onehundredth. Celsius had initially set the freezing point of water at 100° and the boiling point at 0°. This was later reversed after his death. Most countries that have adopted the metric system of measurement use this temperature scale, as it is conveniently broken down into units of 10. In 1948, the Centigrade scale was officially designated the Celsius scale, although some people still use the outdated term.
The most scientific scale in use today is the Kelvin, or absolute, temperature scale. It was devised by British scientist William Thomson (Lord Kelvin), in 1848. Because temperature is a measure of molecular motion, it only makes sense that the zero point of your scale should be the point where molecular motion stops. That is exactly what the Kelvin scale accomplishes. 0 Kelvin (K) is the point at which all molecules stop moving. 0 K is known as absolute zero, which has never actually been reached. In 2003 at MIT, scientists came very close to reaching absolute zero, obtaining a frosty temperature of 4.5 × 10-9 K.
The Kelvin scale is primarily used in science, and temperature must be expressed in Kelvin when solving many equations involving temperature, such as the gas laws. But it tends to be too cumbersome for everyday use, since the freezing point of water is 273 K and the boiling point is 373 K.
Types of Thermometers

The first thermometer in modern times was a crude water thermometer believed to have been invented by Galileo Galilei in 1593. In 1611, Sanctorius Sanctorius, a colleague of Galileo’s, numerically calibrated the thermometer. Many of these first thermometers used wine, as its alcohol content prevented it from freezing and its red color made it easy to read. However, these first thermometers were very sensitive to air pressure, and functioned as much as a barometeras they did as a thermometer. So eventually, all thermometers were constructed of a sealed glass tube that had all the air removed. Because these vacuum tubes were shut off from the outside atmosphere, changes in air pressure would not affect the temperature reading. In 1709, Fahrenheit invented the alcohol thermometer, and in 1714, he invented the first mercury thermometer. All thermometers work according to the same basic principle: objects expand when heated and contract when cooled.
Bulb thermometers
The most common thermometer is the bulb thermometer, which comprises a large bulb filled with a liquid and a narrow glass tube through which the liquid rises. All liquids expand when heated and contract when cooled (with the exception of H2O near its freezing point; ice-cold H2O at 0 °C contracts until 4 °C where it expands like other materials), which explains why the liquid within a thermometer rises as the temperature increases and falls when it decreases. Mercury was the liquid of choice for many years, because it expands and contracts at a very constant rate, making mercury thermometers very accurate. However, because of concerns about mercury toxicity, mercury has often been replaced with alcohol that is colored red. Mercury has a silver color. It freezes at –39 °C, so it cannot be used if temperatures get colder than this.
Bimetallic strip thermometers
Another very common type of thermometer is the bimetallic strip thermometer. This thermometer comprises two different metals, such as copper and iron, which are welded together. Each of the metals used has a different coefficient of linear expansion, or to put it simply, these metals expand at different rates. Connected to this bimetallic strip is a pointer, which points to the correct temperature on the face of the thermometer. Because these metals expand at different rates, when heated, the welded strip of metal will bend. When cooled, it will bend in the opposite direction. A variation of the bimetallic strip thermometer is the thermostat used in homes and automobile engines. These thermostats are made of a thin bimetallic strip, which is fashioned into a coil, making it more sensitive to minor temperature fluctuations.
Infrared Thermometers
A fascinating thermometer is the infrared thermometer. This handheld device is used by simply pushing a button as you point it toward an object. A digital readout tells you the temperature. All objects above absolute zero are emitting infrared radiation (IR)—an invisible (to human eyes) form of electromagnetic energy. The infrared radiation we emit is commonly known as body heat. The infrared thermometer has a lens that focuses the infrared energy into a detector,
which measures the IR intensity and converts that reading to temperature. Infrared thermometers have a wide variety of applications. They are used by firefighters to detect hot spots in buildings and in restaurants to ensure that served food is still warm. Infrared thermometers are also used for determining the temperature of a human body, automobile engines, swimming pools, hot tubs, or whenever a quick surface temperature is needed.
Pop-ups
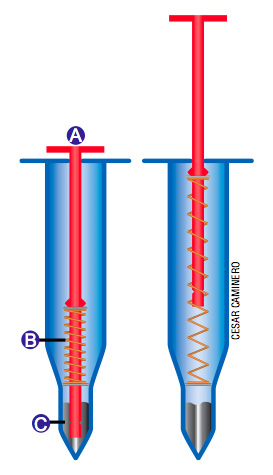
You are cooking that Thanksgiving turkey, and you want to make sure that the inside of the turkey is completely done. To ensure that you are not feasting on undercooked bird, you can use an ingenious device known as the pop-up turkey timer. This instrument is simply stuck into the turkey, and when the turkey is done, a red indicator pops up (A). The little red indicator is spring loaded (B) and is held in place by a blob of solid metal (C). When this metal reaches a temperature of 85 °C, which is the temperature of a fully cooked turkey, it melts, causing the red
indicator to pop up.
This technology is similar to that used in sprinklers found on the ceilings of many buildings, which actually served as the inspiration for the pop-up turkey timers. When a certain temperature is reached, a metal component within these sprinklers melts, activating the sprinkler. By mixing together different metals, a particular alloy can be created with a desirable melting point. Pop-up timers can be purchased for different types of meat, from ham to hens. You can even buy a pop-up timer for steak, which pops up in increments indicating rare to well done.
And Now for Something Completely Different…
Perhaps the most unusual thermometer ever invented is the Galileo thermometer, based on a similar device invented by Galileo. This instrument does not look like a thermometer at all, as it is composed of several glass spheres containing different colored liquids that are suspended in a cylindrical column of a clear liquid. Attached to each of the colored spheres is a little dangling metal tag with an engraved temperature. The temperature is determined by reading the tag on the lowest floating sphere. As the temperature rises, the spheres will begin to fall one by one. When the temperature falls, the spheres will then rise one by one.
The liquid within each glass sphere is composed of either colored water or alcohol. Each of the spheres is of a slightly different mass, and thus a slightly different density, since the volume of each sphere is the same. Each sphere differs in mass by about 0.006 grams. This difference is accomplished by making each tag a slightly different mass. The clear liquid surrounding the spheres is an inert hydrocarbon-based oil, similar to mineral oil. When this liquid is heated, it expands, becoming less dense. Less dense liquids exert a lesser buoyant force, so the most dense sphere will then sink. If the temperature continues to rise, the molecules of the surrounding liquid will continue to spread apart from one another, causing more spheres to fall. As the liquid cools, its molecules come closer together, exerting a greater buoyant force, causing the spheres to rise. The spheres themselves do not expand or contract nearly as much as the surrounding liquid when heated or cooled, since they are composed of glass, which hardly expands at all when heated. Even though it looks nothing like a conventional thermometer, the Galileo thermometer still functions according to the same basic principle as most other thermometers: substances expand when heated and contract when cooled.
What’s the future for Thermometers?
Technology has come a long way since Galileo’s day, but his thermometer to this day has a futuristic look to it. Another futuristic thermometer that is available today is the CorTemp thermometer. Developed by Dr. Leonard Keilson of the Applied Physics Laboratory of the Johns Hopkins University in conjunction with NASA, the CorTemp thermometer is swallowed, allowing accurate temperature readings while it travels through, or is stationed at some particular spot in the body.
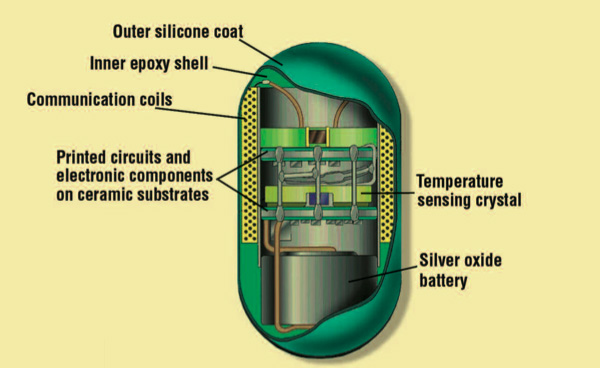
The probe is enclosed in a small pill that is taken internally, while the temperature readings are recorded on a device that is monitored externally. No matter what device you use to take your temperature when you have a fever, none will make you feel better. But in this technologically advanced world today, your choice of thermometer might bring you a bit of welcomed distraction while measuring the average kinetic energy of your body’s molecules.
Download Article

